How Many Bonds Can Silicon Make
The simple logic is that 4 4 8. Well actually it can form more than four bonds.
The dianion S i F 6 2 is well-known.

. Silicone rubber is one of the most challenging substrates to bond and there are only a few adhesives that can make a strong bond. Based on this fact you can conclude that silicon like carbon could form four covalent bonds. A nitrogen atom forms three covalent bonds.
The element silicon would be expected to form 4 covalent bonds in order to obey the octet rule. Silicon can appear as a heteroatom in many organic molecules for instance Si CH34 which is the reference compound for 1H NMR spectroscopy. Thats why we can safely predict the number of bonds most elements in the main groups Group 1 2 13 17 make but not the elements that are in the transition metals block.
When figuring out whether to place a double or triple bond you should always look at the number of valence electrons present as well as the number of bonds a central atom is likely to form. Silicon has the same valence configuration as carbon so like carbon it forms 4 bonds. Iodine can form 1 3 5 or 7 covalent bonds.
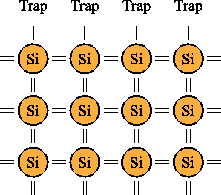
This valence electron participates in the formation of bonds with atoms of other elements. In the silicon crystals that form the backbone of the electronics industry each silicon atom forms covalent bonds with four other silicon atoms sharing one of its electrons and receiving a shared electron in return from each of the four neighbors. This is a special case as with hydrogen.
And germanium tin lead and flerovium are below it. In general silicon has a chemistry parallel to carbon as we would expect from the position of silicon on the Periodic Table. That also means it can form multiple number of bonds.
A new study is the first to show that living organisms can be persuaded to make silicon-carbon bondssomething only chemists had done before. When elements are in the same column they act in similar ways. Tungsten is a tough one because its a transition metal that means it has variable oxidation number.
The C represents the carbon nucleus center and the 4 lines or sticks going up down left and right represent the bonds. Silicon has the same number of electrons in its outer shell meaning that it can form four bonds just like carbon. In the diagram above we show carbon making 4 bonds.
Predict the molecule formed from carbon and chlorine. Silicon atom needs four more electrons to become most stable. The number of valence electrons an atom possesses determines how many covalent bonds it can form.
Predict the molecule formed from silicon and oxygen. Silicon atoms form bonds by sharing electrons with oxygen atoms. Answered 3 years ago Author has 202K answers and 82M answer views.
Silicon can bind readily to itself to make Si-Si bonds just like carbon can make C-C bonds. How many bonds does silicon tetrachloride SiCI4 have. Group of answer choices 3 1 1 See answer it is 4 Advertisement Advertisement allisonmarieross317 is waiting for your help.
So in order to be stable Silicon Sineeds to form four covalent bonds. The electron configuration of oxygen shows that the valence electrons of oxygen are six. It forms four bond View the full answer.
How many covalent bonds can iodine form. Even though nitrogen has five valence electrons it is unable to form five covalent bonds. It is a member of group 14 in the periodic table.
Since nitrogen has five valence electrons and bonds it uses three of its five valance electrons for bonding. The same idea would apply to silicon Si another element in column 4. As a carbon analogue silicon forms four covalent bonds with hydrocarbyl groups.
How many total bonds can one atom of this metalloid make with other atoms. These only need 2 valence electrons. How many covalent bonds does a single atom form in a silicon crystal.
It needs to make 4 bonds to get an octet. How do you know if a covalent bond is single double or triple. Physics questions and answers.
1 Silicon Si it usually forms four bonds with all compounds except fluorine. Scientists at Caltech bred a bacterial protein to. To draw a Lewis structure for a polyatomic ion begin by calculating A the available electrons and N the needed electrons.
Helium already has 2 so it will typically make zero bonds 26-Sep-2016. How many bonds can lead Pb form. If you look at the periodic table youll see that silicon Si is directly below carbon C.
How many bonds can helium form. Another good way to know whether to use. Silicon is a chemical element with the symbol Si and atomic number 14.
Si is a nonmetal in group 4A and therefore. Add your answer and earn points. Silicon atom forms four covalent bonds with the four neighboring atoms.
Two oxygen atoms and one silicon atom make silicon dioxideSiO 2 compounds by sharing electrons. So silicon creates two double bonds with two separate oxygen O atoms. Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell.
It is also very abundant comprising much of the rock that is beneath your feet. How many electrons does silicon 3 have. Silicon is made up of 14 electrons 14 protons and in most cases 14 neutrons.
Predict the molecule formed from aluminum and oxygen. It is a hard brittle crystalline solid with a blue-grey metallic luster and is a tetravalent metalloid and semiconductor. Carbon is above it.
In covalent bonding each valence electron is. How many bonds can barium Ba form. One option that has been successful in other silicone rubber applications is 3M Scotch-Weld Plastic Rubber Instant Adhesive PR100 with 3M Scotch-Weld Instant Adhesive Primer AC79.
WILL MARK BRAINLEST The image above shows an electron dot diagram for silicon.

Silicon Atom Physics Tutorial Semiconductor Physics Semiconductors

Covalent Bonding In Silicon Dioxide Chemistry Covalent Bonding Chemistry Revision
![]()
Intrinsic Semiconductor Covalent Bonding In Silicon And Germanium
No comments for "How Many Bonds Can Silicon Make"
Post a Comment